Data Protection Solutions For Life Sciences - Newsletter #33

In this Newsletter
Summary
This edition covers major regulatory developments, including new EU guidance on labelling AI generated content, Spain’s practical AI Act compliance guides, and updated FDA and NIST frameworks shaping AI, cybersecurity, and medical device oversight. We also explore how AI is transforming life sciences, from FDA cleared AI tools for sleep apnea and liver disease trials to growing use of real world data and AI driven drug discovery partnerships. Finally, we look at healthcare innovation and risk, with new funding for clinical trial access, real time patient tracking pilots, and a reminder of rising cybersecurity threats following a major ransomware attack on a research firm.
Regulations & Guidelines

EU Releases First Draft Rules on Labelling AI-Generated Content
The European Commission has published the first draft of a voluntary Code of Practice to help organisations comply with the AI Act’s transparency obligations. The Code clarifies how AI-generated and manipulated content, including deepfakes, should be marked and labelled ahead of mandatory enforcement in 2026. Stakeholders are invited to provide feedback as the framework takes shape.

NIST Publishes New Cybersecurity Framework for AI
NIST has released a preliminary draft of its Cybersecurity Framework Profile for Artificial Intelligence, outlining how organizations can manage AI-related cyber risks and leverage AI for defense. Built on the NIST CSF 2.0, the profile addresses securing AI systems, defending against AI-enabled threats, and strengthening cyber resilience, with public comments open through January 2026.

Spain Publishes Practical AI Act Compliance Guides
Spain’s AI supervisory authority has released a comprehensive set of non-binding guides to help organizations prepare for compliance with the EU AI Act. Covering topics from risk management and transparency to post-market monitoring and technical documentation, the resources offer practical direction ahead of harmonised EU standards.

AI-Ready 510(k): How FDA Uses Elsa and eSTAR to Streamline Submissions
The FDA is integrating AI tools like Elsa to streamline the 510(k) medical device submission process while maintaining human oversight. Key changes include mandatory eSTAR submissions, which improve information organization, and AI automation of tasks such as technical screening and gap identification. Developers should prepare "AI-ready" submissions using structured formats, clean data, and minimal redundancy to maximize these benefits.
AI and TechBio
Hospitals are Deleting AI Scribe Data and Researchers are Alarmed
New research raises concerns that hospitals and AI medical scribe providers are routinely discarding visit transcripts that could reveal accuracy gaps and patient safety risks. While AI scribes promise efficiency gains, experts warn that deleting underlying data may prevent meaningful evaluation of errors and clinical impact.

Why AI Needs Physics to Unlock the Next Breakthroughs in Drug Discovery
As AI reaches its limits in drug discovery, researchers are increasingly turning to physics-based models to better understand molecular behavior. The convergence of AI and physical sciences is emerging as a critical path toward more reliable predictions and transformative pharmaceutical innovation.
Novartis Bets Big on AI Partnership for Atopic Diseases
Novartis has entered a major AI-driven drug discovery partnership with UK biotech Relation Therapeutics, targeting atopic diseases. The deal underscores Big Pharma’s growing reliance on AI platforms and patient-derived data to identify first-in-class drug targets and accelerate R&D pipelines.
FDA Clears AI-Driven CPAP Personalization for Sleep Apnea
The FDA has cleared ResMed’s Smart Comfort AI, a machine-learning system designed to personalize CPAP therapy for sleep apnea patients. Trained on over 100 million sleep records, the technology aims to improve comfort, adherence, and outcomes, with a limited U.S. rollout planned for 2026.
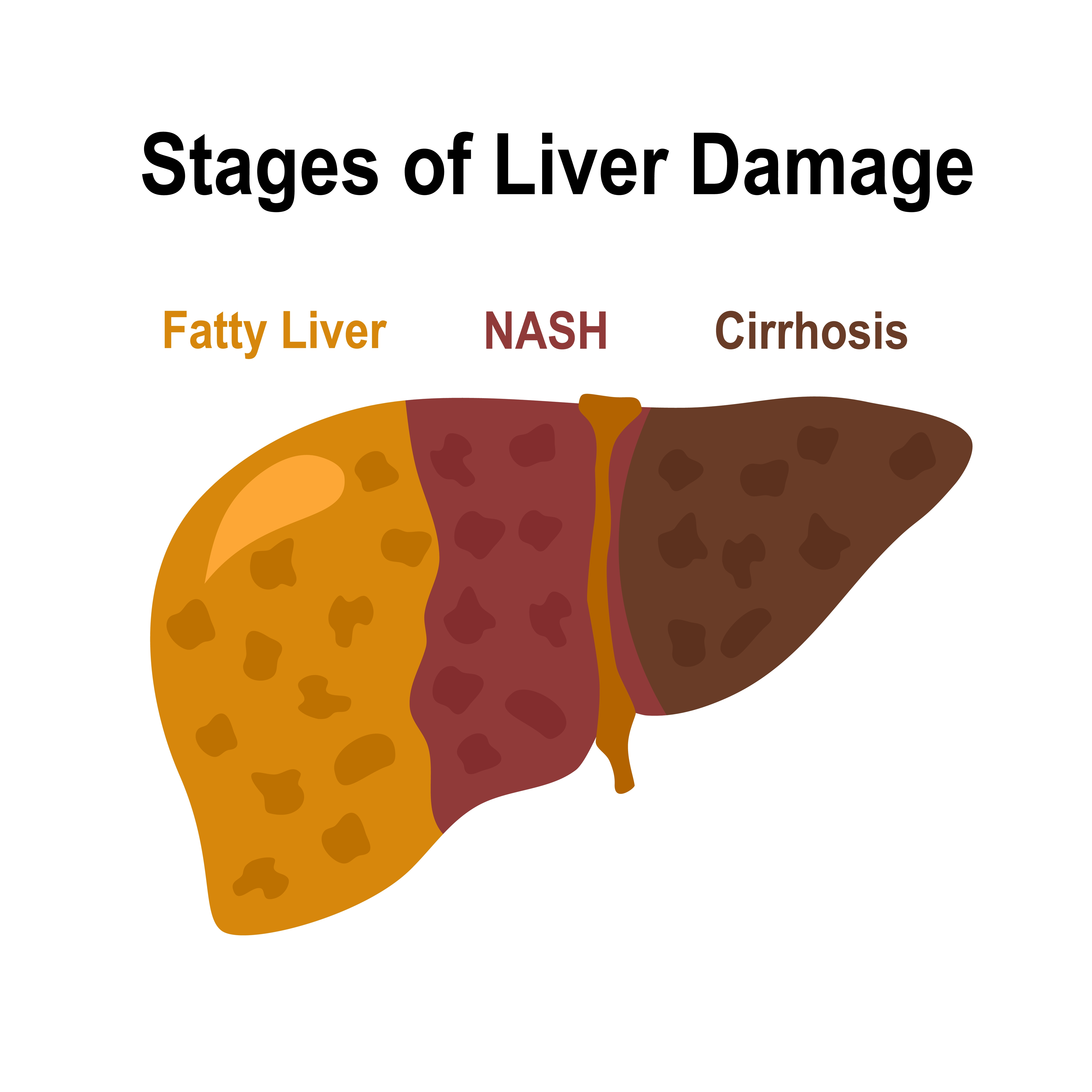
FDA Qualifies First AI Tool for Evaluating MASH in Drug Trials
The FDA has qualified AIM-NASH, the first AI-powered tool approved to assist drug developers in evaluating fatty liver disease during clinical trials. By standardising biopsy image analysis with AI and expert review, the tool could speed development in a historically challenging disease area.
BioTech, Healthtech and Healthcare

Trial Library Raises $10M to Expand Clinical Trial Access
Trial Library has secured $10 million to scale its patient-navigation platform that helps match cancer patients to clinical trials through insurers. By combining human navigators with digital tools, the company aims to improve trial access, equity, and enrolment beyond academic medical centers.
Startup Tests Real-Time Patient Tracking for Stroke Transport
Digital health startup iAVC is launching a regulatory pilot to assess whether real-time geolocation of stroke patients during transport can improve care coordination. The experiment could reduce delays and improve outcomes while minimising operational burden for emergency care teams.
Data Breach & Cybersecurity

Drug Research Firm Inotiv Hit by Major Ransomware Attack
Pharmaceutical research company Inotiv has confirmed a ransomware attack that exposed employee and partner data affecting thousands of individuals. The incident highlights ongoing cybersecurity risks in life sciences and the growing impact of data breaches on research organisations and their collaborators.
Podcasts
iliomad's News

Looking forward to 2026 !
The iliomad Health Data team would like to wish you a wonderful 2026. On our side, we are expanding our team, services, and geographical coverage to make iliomad a leading compliance partner in the life sciences, supporting biotech, healthtech, and techbio companies with their worldwide operations.

Sign up for our newsletter
We like to keep our readers up to date on complex regulatory issues, the latest industry trends and updated guidelines to help you to solve a problem or make an informed decision.

Data Protection Solutions For Life Sciences - Newsletter #33
Regulators advance AI rules as innovation accelerates amid governance and safety concerns

Data Protection Solutions For Life Sciences - Newsletter #32
Privacy reforms advance; AI boosts drug discovery, mental health care, ADCs, healthspan.

Data Protection Solutions For Life Sciences - Newsletter #31
November spotlight: global AI, privacy, and healthtech reforms driving stricter regulatory compliance worldwide.



